原标题:惯用左手的医学美容,惯用右手的创新药物,这家公司日新月异地发展,今天它每天都有限制
概括
[Left-handedmedicalbeautyright-handedinnovativedrugsthiscompanyhassoareddayafterdayandtodayithasadailylimit!】BeforetheSpringFestivalthesharepriceofAmictheleadingmedicalbeautycompanyexceeded1000yuanwhichdrovetheriseoftheentiresectorOnthefirsttradingdayaftertheholidaythemedicalbeautysectorremainedenthusiasticOnFebruary18EastChinaPharmaceuticalLushangDevelopmentJingfengPharmaceuticalandLangzisharessurged
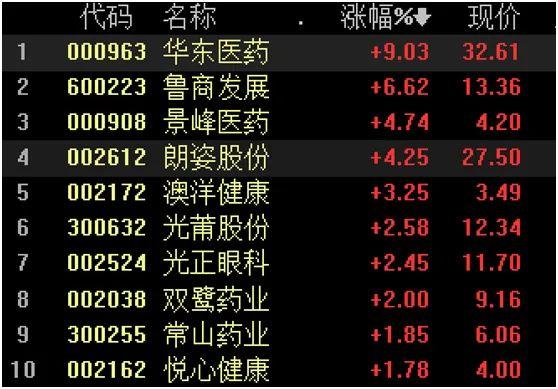
The concept of medical beauty continues to strengthen,Huadong MedicineSoaring 9%.
Before the Spring Festival, the leading medical beautyAmicThe stock price exceeded 1,000 yuan, which drove the rise of the entire sector. On the first trading day after the holiday, the medical beauty sector remained enthusiastic. February 18,Huadong Medicine、Lushang Development、Jingfeng Pharmaceutical、Longzi sharesAnd so on soaring.

among them,Huadong MedicineBecause in the past two daysannouncementTwo important transactions in the fields of medical aesthetics and innovative drugs were concluded, and the stock price moved higher and reached a daily limit.As of the close at noon, it was up 9.03%, and the transaction amount reached 2.282 billion yuan, at 32.61 yuan per share.Market value57.1 billion yuan. It is worth noting that Huadong Medicine has risen continuously in the previous three trading days, with a cumulative increase of over 30%.
In recent years, as the beauty economy has gained momentum, the medical beauty industry has ushered in a golden period of vigorous development.According to the data of the “Insight White Paper on China’s Medical Beauty Industry in 2020” released by iResearch, China’s medical beauty industry in 2019marketThe scale reached 176.9 billion yuan, with a growth rate of 22.2%; in 2019, China’s medical beauty users were 13.672 million, and it is predicted that medical beauty users will reach 25.483 million in 2023.
FromindustryFrom the chain point of view, Huadong Medicine,AmicWaitthe companyIn medical beautyIndustry chainUpstream.Haitong InternationalIt is believed that upstream manufacturers are the best subdivision track in the medical aesthetics industry chain, with high market concentration and a good competitive landscape.interest rateHigh; short-term focus on channels and sales, medium and long-term focusproductThe R&D layout pipeline conforms to the logic of the strong.The R&D and iteration capabilities of the product areenterpriseThe most important moat.
Huadong Medicine acquires Spanish medical beauty equipment company for 85 million Euro
On the evening of February 17, Huadong Medicine issued an announcement stating that the company passedWholly-owned subsidiaryEquity consideration of 65 million euros and a sales milestone of up to 20 million eurospayment, Acquired HighTechnology Products, SLU, a Spanish energy-source medical beauty equipment company. 100% equity.

The announcement shows that High Tech’s business covers the two major medical and aesthetic fields of body shaping and skin repair, and has leading independent core technologies. The main products include a series of products such as cryo-fat and laser hair removal. In 2019, its cryo-fat products ranked second in the market share of EMEA (Europe, Middle East, and Africa) shaping and firming equipment.
Huadong Medicine stated that this acquisition will further enrich the medical aesthetics product pipeline and expand business areas, broaden energy source medical aesthetics devices, realize the company’s full coverage of non-surgical mainstream medical aesthetics products, and open up “minimally invasive + non-invasive” medical aesthetics New era; formation containsDifferentiationThe comprehensive product cluster of sodium hyaluronate full product portfolio, collagen stimulant, type A botulinum toxin, implantation line, and energy source equipment can better meet the needs of beauty seekers.
It is reported that around 2017, energy source medical beauty equipment manufacturers have undergone acquisitionsmergerThe tide, currently listed foreign companies includeJohnsonAnd Bausch & Lomb, listed domestic companies include Qizhi Laser and Fu Rui Medical (acquired Alma).China SecuritiesSecurities released a few days agoResearch reportSaid according toNew oxygenAccording to the released “White Paper on Medical Beauty Industry in 2019”, the proportion of the number of treatments in the energy source medical beauty project is only 5%, which is about double the space in the future compared with other countries. Therefore, the market space of this segmented track is relatively limited in the entire medical beauty industry.
The medical beauty track has attracted much attention from the capital market in recent years. As one of the leading companies, Huadong Medicine has continuously expanded its medical beauty territory through domestic and foreign acquisitions in recent years. It currently has five production bases in the Netherlands, France, the United States, Switzerland and Bulgaria. .
In 2018, Huadong Medicine acquired Sinclair, a British company that focuses on beauty lines, long-acting microspheres, and hyaluronic acid. Sinclair is currently its global operation center for its medical beauty business. In 2019, it became a shareholder of American medical beauty company R2, and its main product is frozen freckle medical equipment. In August 2020, Huadong Medicine and Jetema, a listed South Korean company, on the exclusive use of its type A botulinum toxin products in ChinaproxyRight to sign an agreement. In October 2020, it announced the acquisition of Kylane, a Swiss hyaluronic acid company, and obtained its newprofitableThe exclusive global license for Kaine Hyaluronic Acid Filler (Hyaluronic Acid) products has been approved for EU marketing.
Huadong Medicine plans to introduce global innovative drugs under research at a cost of US$189 million to increase its layout in the field of autoimmunity
On the morning of February 18, Huadong Medicine announced that the company’s wholly-owned subsidiary Hangzhou Sino-American Huadong Pharmaceutical Co., Ltd. and Provention Bio, Inc of the United States.Reached exclusive clinical development andbusinessSino-US East China obtains two clinical indications of Provention Bio’s product under development-the bispecific antibody PRV-3279 (for the treatment of systemic lupus erythematosus in the first phase of the US clinical trial, used to prevent or reduce the immunogen of gene therapy Sex is in preclinical research in the United States), inGreater ChinaExclusive clinical development and commercialization rights. Sino-US East China will pay Provention Bio a down payment of US$6 million, R&D and production support funding of US$11.5 million, a milestone payment of up to US$172 million, and an agreed tiered, double-digit net sales commission fee.

According to the announcement, Provention Bio was established in 2016, established and continued under the laws of the state of Delaware, the registered address is in Delaware, the main office address is in Red Bank, New Jersey, the United States, in July 2018 in the United States NASDAQ securities It is listed on the stock exchange, and the current market value is about US$950 million (data on February 9, 2021). As of September 2020, Provention Bio has total assets of US$152 million and total liabilities of US$16.404 million. Realized from January to September 2020Operating income$0,Net profit-660.5 million US dollars.
The company is a clinical-stage biopharmaceutical company dedicated to the development and commercialization of innovative treatments for blocking and preventing immune-mediated diseases. There are several innovative products in the development stage, and its PRV-031 (Anti-CD3 monoclonal antibody) is used to delay or prevent type 1 diabetes in high-risk groups, and has submitted a biological product license application (BLA) to the US Food and Drug Administration (FDA). The PRV-015 (anti-IL-15 monoclonal antibody) developed by Provention Bio and Amgen Inc in the United States for the treatment of celiac disease is undergoing phase 2 clinical trials in the United States.
The target product PRV-3279 of the cooperation between the two parties is used for the prevention of systemic lupus erythematosus or reducing the immunogenicity of gene therapy. It is currently undergoing trials for two indications in the United States. Among them, SLE indications have completed two phase 1 trials of 1a and 1b; the immunogenic indications for preventing or reducing gene therapy are undergoing preclinical trials.
Huadong Medicine stated that through this cooperation, the company will establish a research and development partnership with Provention Bio in the field of autoimmune disease treatment.borrowWith its rich clinical development experience, the two parties look forward to continuing to carry out broader and deeper cooperation in the development of innovative drugs in the field of autoimmunity. This cooperation will have a positive impact on the company’s future R&D and innovation capabilities and the introduction of international product cooperation. In the future, the company will continue to increase its layout in the field of autoimmunity. Based on a large number of unmet clinical needs, relying on the company’s existing R&D platform to increase R&D investment in antibody products, strengthen and deepen the product innovation chain and industrial ecological chain, and continue to enrich The R&D pipeline will ultimately realize Huadong Medicine’s leading market competitiveness and international layout in the field of autoimmune disease treatment.
(Source: Securities Times Net)
(Editor in charge: DF358)
Solemnly declare: The purpose of this information released by Oriental Fortune.com is to spread more information and has nothing to do with this stand.